Findings
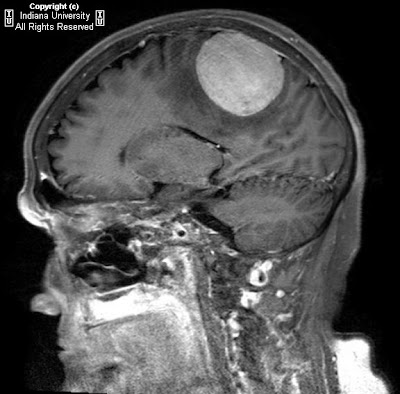
Figure 1, Figure 2, and Figure 3 demonstrate increased signal in the region of the bilateral basal ganglia, left thalamus, subthalamic region and brainstem. Diffusion-weighted images shows an area of restricted diffusion (Figure 2 and Figure).
Figure 4 shows contrast enhancement on T1WI in the left basal ganglia and focal meningeal enhancement.
Figure 5 single voxel MR spectroscopy within the region of interest demonstrates increased choline, slightly decreased NAA, and presence of lactate peak.
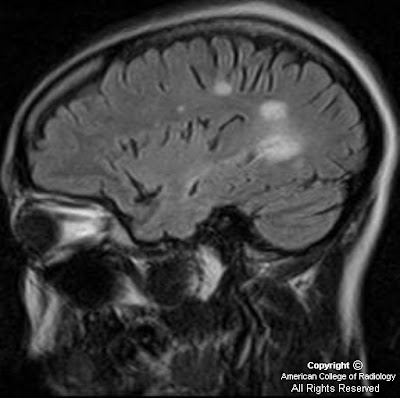
Figure 6, Figure 7, and Figure 8 Sagittal and axial T2 and axial T1 post contrast images show an abnormal long segment of high T2 signal in central cervical cord with subtle contrast enhancement.
Diagnosis: Vasculitis/Behcet disease
CNS vasculitis is a heterogeneous group of disorders characterized by nonatheromatous inflammation and necrosis of blood vessel walls. Arteries and veins are affected; it can involve intracranial vessels of any size.
A variety of systemic inflammatory diseases can cause vascular inflammation and stroke. Neuro-Behcet is a type of CNS vasculitis. Behcet disease is a chronic, relapsing, inflammatory disease characterized by presence of recurrent and usually painful mucocutaneous ulcers, genital lesions, ocular lesions, neurologic manifestations and cutaneous manifestations. Behcet is uncommon in the United States.
Neurologic manifestations are seen in approximately 10-25% of patients with Bechet disease. CNS manifestation results from arterial or venous thrombosis. Neuro-Behcet disease (NBD) usually shows three clinical patterns: A brainstem syndrome, a meningomyelitic syndrome and an organic confusion syndrome.
The typical MRI findings are multiple focal T2 signal abnormalities, restricted diffusion in the acute phase of disease, and patchy vascular and leptomeningeal enhancement. The most common site of involvement is the brainstem, followed by white matter, internal capsule and basal ganglia or thalamus. Brainstem atrophy is one of the manifestations of chronic NBD. Meningeal involvement is a less frequent finding. Follow up studies show change in site, size and shape of the lesions. Cerebral venous thrombosis is seen in approximately 0.6 to 10% of Behcet disease. The spinal cord involvement is rare. It usually shows long segment lesions demonstrating high signal in T2 WI, which may show patchy enhancement. Thoracic and cervical cords are usual sites of involvement.
In the largest series to date, the clinical features and outcomes of 200 patients with Behcet disease and neurologic involvement were reported. On average, a period of approximately 5 to 6 years elapsed between the onset of the earliest non-neurologic symptoms of Behcet disease and the appearance of neurologic symptoms or findings. In a small percentage of Behcet, neurologic findings may appear concurrently or precede non-neurologic features.
The prognosis varies with the type of neurologic process. Those with dural venous thrombosis or other non-parenchymal processes are less likely to have recurrent disease, disability, or premature death. By comparison, patients with parenchymal disease have a worse outcome.