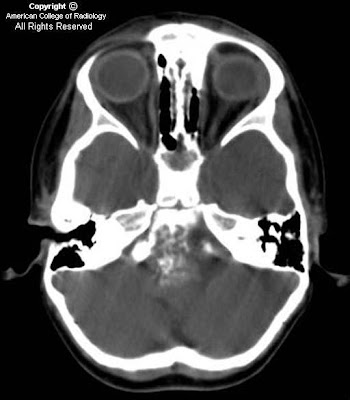
Findings
There is a small left frontal extra-axial hypodense fluid collection with associated thinning of the calvarium. No significant mass effect on the brain parenchyma. No acute intracranial abnormalities.
Differential diagnosis:
- Arachnoid cyst
- Chronic subdural hematoma
- Subdural hygroma
- Epidermoid cyst
- Prominent CSF space
- Porencephalic cyst
- Neuroglial cyst
Diagnosis: Arachnoid cyst
Key points
An arachnoid cyst is a benign, intra-arachnoid, CSF-filled cyst that does not communicate with the ventricular system. Arachnoid cysts are fairly common, accounting for approximately 1% of all intracranial masses. They are often asymptomatic and are found incidentally, but are twice as common in imaging performed for history of seizure. If present, symptoms vary with the size and location of the cyst and can include, headache, sensorineural hearing loss, dizziness, and even obstructive hydrocephalus. Arachnoids cysts are usually stable in size but have been known to slowly enlarge. No treatment is usually required, but resection, fenestration, or shunt placement may be performed for serious symptoms.
Radiology
In general: Arachnoid cysts may appear anywhere in the cranium, although the middle cranial fossa is the most common location. They can vary in size from only a few millimeters to ten centimeters or more. They typically displace cortex and may even "buckle" the gray-white border.
CT: CSF density, usually associated thinning/remodeling of adjacent bone, no enhancement with contrast
MR:
- T1WI - isointense with CSF, sharp margins
- T2WI – isointense with CSF
- FLAIR – suppresses completely
- DWI – no restricted diffusion
- T1+C – no enhancement